PARADIM Highlight #96—External User Project (2024)
Julia Mundy (Harvard), Antia Botana (ASU), and collaborators
Since the discovery of high-temperature superconductivity in cuprates, work has also focused on materials with the same structures including the nickelates, as both Cu2+ and Ni1+ have 27 electrons. The superconducting nickelates include (La,Sr)NiO2 (equivalent to Rn+1NinO2n+2 with n = ∞ and R = La or Sr) and Rn+1NinO2n+2 with n = 5 and R=Nd or equivalently Nd6Ni5O12.
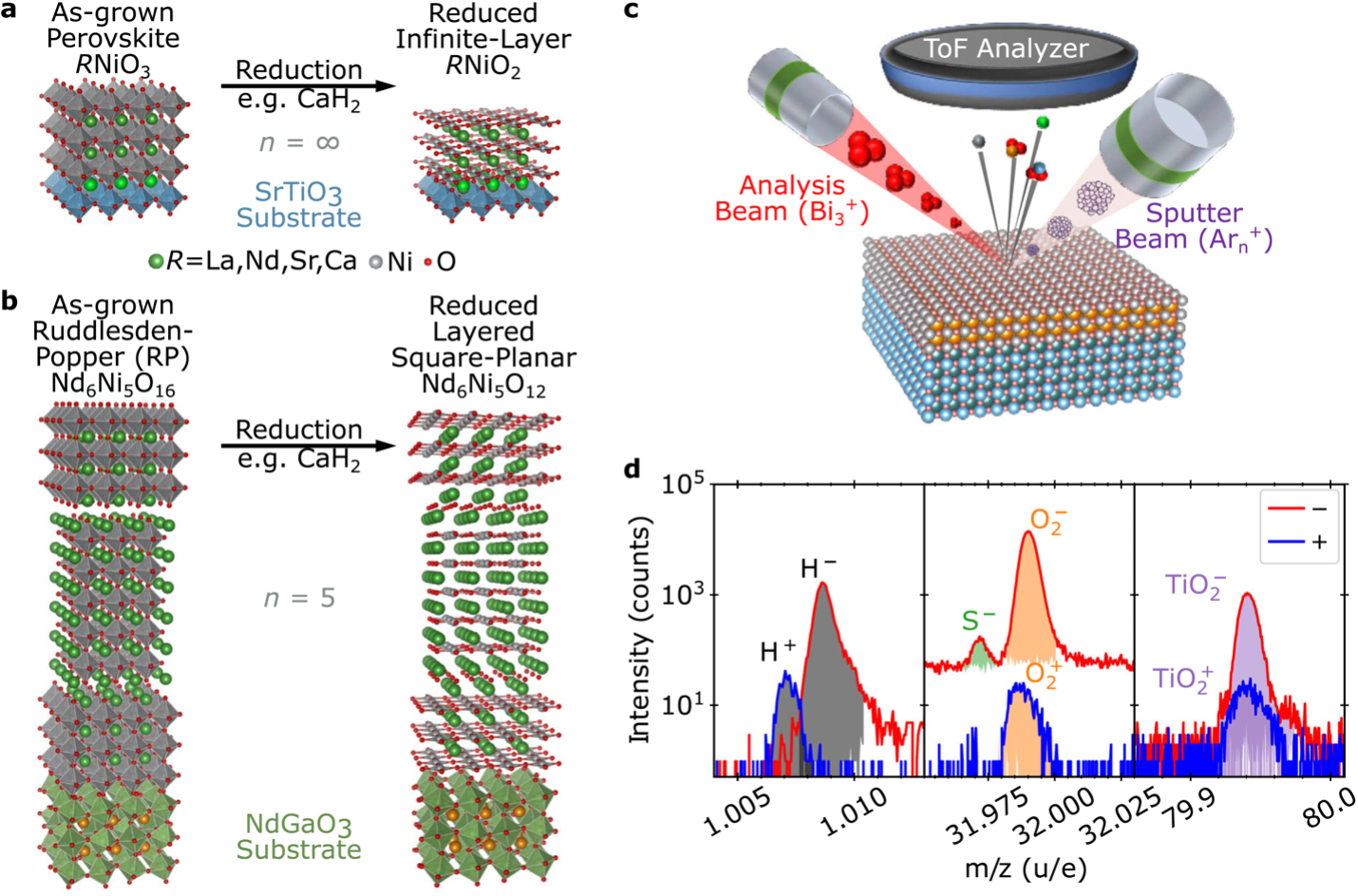
Figure 1: Schematic crystal structures of precursor phase and reduced to n = ∞ (a) and n = 5 (b) layered square-planar nickelate compounds. c) Schematic of the ToF-SIMS measurement technique. d) SIMS spectra measured separately for positive and negative ions are analyzed by identifying peaks by mass-to-charge ratio (m/z) and extracting integrated area.
Beyond superconductivity, the layered nickelates pose a synthesis challenge as a two-step fabrication process is required. An oxygen-rich precursor material is first grown by traditional thin film techniques and then oxygen is removed without collapsing the structure using H2, NaH, or CaH2. As these reagents contain hydrogen, the question arises whether hydrogen incorporation into the structure is a prerequisite for superconductivity.
Here, PARADIM users from Harvard and Arizona State Universities and their collaborators searched for hydrogen across a wide range of superconducting and non-superconducting layered nickelate films, with different cation and dopant chemistry, structures, growth methods, reduction conditions, and crystalline quality. They did not find significant concentrations of hydrogen in superconducting films and were also unable to use excessive reduction temperature or time to force significant amounts of hydrogen into these structures. The team’s DFT calculations further corroborate that superconductivity does not rely on hydrogen.
Superconductivity in nickelates has been pursued ever since the discovery of the cuprates, but it was not until 2019 that it was demonstrated in thin films of the infinite-layer compound NdNiO2 via hole doping with Sr. This discovery introduced a novel family of layered nickelate superconductors that has now been extended to include the Pr- and La- analogs of the infinite-layer compound as well as the five-layer material Nd6Ni5O12. While superconducting nickelates exhibit many interesting phenomena, they also represent a unique materials synthesis challenge. In general, layered square-planar nickelates cannot be synthesized directly, instead requiring a two-step fabrication method wherein an oxygen-rich precursor material is grown by traditional thin film deposition methods and then topotactically reduced. Typically, the reduction is performed via a thermal anneal employing a chemical reducing agent and oxygen sink, such as H2, NaH, or CaH2. One of the most pressing open questions is the degree to which the reduction process incorporates hydrogen into the nickelate film, and whether hydrogen is important in stabilizing superconductivity.
Despite 38 years of high-temperature superconductivity, it is still not understood. Clues about it come from establishing where it exists and does not exist. In this regard, the discovery of superconducting nickelates with the same electron count on the Cu2+ as on Ni1+ (both have 27 electrons) and the same crystal structures is enriching our knowledge. The synthesis of the nickelates (in contrast to the cuprates) involves reduction using a species containing hydrogen. This has raised the question of whether hydrogen is essential for superconductivity in the nickelates. This question is answered by this work and the answer is that hydrogen is not essential.
This work made use of two PARADIM user facilities. The n = 5 (Nd6Ni5O18) and n = ∞ (NdNiO3) precursor films, that were subsequently reduced to Nd6Ni5O12 and NdNiO2, respectively, were grown using the PARADIM MBE and imaged using PARADIM’s electron microscopy facility.
The work was initialized by PARADIM user Prof. Julia Mundy, Harvard University and includes major contributions from the PARADIM In-House Research Team, the Arizona State University, the National Institute of Standards and Technology (NIST), National University of Singapore, and University of Southern California.
P.P. Balakrishnan, D.F. Segedin, L.E. Chow, P. Quarterman, S. Muramoto, M. Surendran, R.K. Patel, H. LaBollita, G.A. Pan, Q. Song, Y. Zhang, I. El Baggari, K. Jagadish, Y.-T. Shao, B.H. Goodge, L.F. Kourkoutis, S. Middey, A.S. Botana, J. Ravichandran, A. Ariando, J.A. Mundy, and A.J. Grutter, "Extensive Hydrogen Incorporation is Not Necessary for Superconductivity in Topotactically Reduced Nickelates," Nat. Commun. 15, 7387 (2024). DOI: https://doi.org/10.1038/s41467-024-51479-3
D.F.S., G.A.P., and J.A.M. acknowledge support from the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under Award No. DE-SC0021925. L.E.C. and A.A. acknowledge support from the Ministry of Education (MOE), Singapore, under its Tier-2 Academic Research Fund (AcRF), Grants No. MOET2EP50121-0018 and MOE-T2EP50123-0013, and the SUSTech-NUS Joint Research Program. J.R. and M.S. acknowledge support from an ARO MURI program with award no. W911NF-21-1-0327, and the National Science Foundation of the United States under grant number DMR-2122071. R.K.P. and S. Middey acknowledge MHRD, Government of India for financial support under the STARS research funding scheme (grant number: STARS/APR2019/PS/156/FS). J.A.M., A.S.B., and H.L. acknowledge support from NSF grant no. DMR-2323971. I.E. and Y.Z. were supported by the Rowland Institute at Harvard. K.J. and Y.T.S. acknowledge support from USC Viterbi startup funding and the USC Research and Innovation Instrumentation Award. B.H.G. and L.F.K acknowledge support from the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM) and the Packard Foundation. Materials growth and electron microscopy were supported in part by PARADIM under NSF Cooperative Agreement no. DMR-2039380. Focused ion beam sample preparation was performed in part at the Harvard University Center for Nanoscale Systems (CNS); a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. ECCS-2025158. Transmission electron microscopy was carried out in part through the use of MIT.nano’s facilities. Electron microscopy data were acquired in part at the Core Center of Excellence in Nano Imaging at USC. Electron microscopy was performed in part at the Cornell Center for Materials Research (CCMR) Shared Facilities, which are supported by the NSF MRSEC Program (No. DMR-1719875). P.P.B. and P.Q. received funding from the NRC RAP. G.A.P. acknowledges additional support from the Paul and Daisy Soros Fellowship for New Americans. D.F.S. and G.A.P acknowledge support from the NSF Graduate Research Fellowship Grant DGE-1745303. We thank Kyuho Lee for insightful discussions. We also thank Kerry Sieben for X-ray assistance. We thank Hanjong Paik for supporting the growth of the n = 5 superconducting sample. Research was performed in part at the NIST Center for Nanoscale Science and Technology. Certain commercial equipment, instruments, software, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identifications are not intended to imply recommendation or endorsement by NIST, nor it is intended to imply that the materials or equipment identified are necessarily the best available for the purpose.







